| Corrosion of concrete structures by general atmospheric environment |
The general atmospheric environment refers to only normal atmospheric conditions (such as carbon dioxide, oxygen, etc.) and temperature and humidity (moisture), without the influence of freezing, thawing, chloride, and other chemical corrosive substances. The corrosion of concrete structures in general atmospheric environment is mainly caused by steel corrosion caused by carbonization, which is commonly seen in industrial and civil buildings. CO is a natural component in the atmosphere, and for concrete structures, CO is the main cause of concrete carbonation and steel corrosion. At present, the volume of CO in the atmosphere accounts for about 0.03% of the total volume of the atmosphere. Human life and industrial activities release CO, which accounts for about 3% of the natural release, but does not cause significant changes in the concentration of CO in the atmosphere. According to statistics, the concentration of CO in the air increases at an annual rate of 0.4%, so overall the concentration of CO remains at 0.03%. However, human activities may cause a significant increase in CO concentration in partially enclosed environments, such as underground garages, factories, shopping malls, etc.
Concrete carbonation refers to a multiphase physicochemical process in which CO or certain acidic gases in the environment come into contact with the surface of concrete exposed to the air and continuously diffuse into the interior of the concrete, reacting with alkaline hydrates (such as CaO) in the concrete to produce carbonic acid or other substances. Carbonization causes a decrease in pH in the pore solution of concrete, tending towards neutralization. When the pH in concrete decreases to a certain extent, it will damage the passive film of steel bars in the concrete, causing steel bar corrosion. Steel bar corrosion will also lead to adverse consequences such as cracking of the concrete protective layer, damage to the bond between steel bars and concrete, and reduced structural durability. In addition, carbonation makes concrete brittle and reduces the ductility of components.
Generally, the general atmospheric environment is classified based on the specific environment in which the structure or component is located, including indoor environment, outdoor environment, dry, humid, permanently submerged, and alternating dry and wet conditions. The environmental factors that affect concrete carbonation mainly include the following.
1. CO: concentration
CO: The higher the concentration, the faster the carbonization. The concentration of CO in the actual atmosphere varies with time and location. In the northern hemisphere, due to plant respiration, the concentration of CO also undergoes periodic changes: it increases in autumn and winter, and decreases in spring and summer. Of course, this change is completely invisible near the equator. In addition, the concentration of CO in densely populated large cities and industrial areas with high CO emissions can reach over 0.05%, while in rural areas it is greatly reduced; Less during the day and sunny days than at night and rainy days; On land, it is larger than on the ocean. Except in tunnel engineering where air exchange is slow, air flows. In a normal atmospheric environment (different from environments with less air exchange such as tunnels), small regional differences in CO concentration have relatively little impact on the depth of carbonization.
2. Environmental temperature
The diffusion rate of gas and carbonization reaction are greatly affected by temperature, and as the temperature increases, the carbonization rate accelerates. Experimental studies have shown that under the conditions of CO concentration of 10% and relative humidity of 80%, the carbonization rate at a temperature of 40C is twice that of 20C; Under the conditions of CO concentration of 5% and relative humidity of 60%, the carbonization rate at a temperature of 30C is 1.7 times that of 10C.
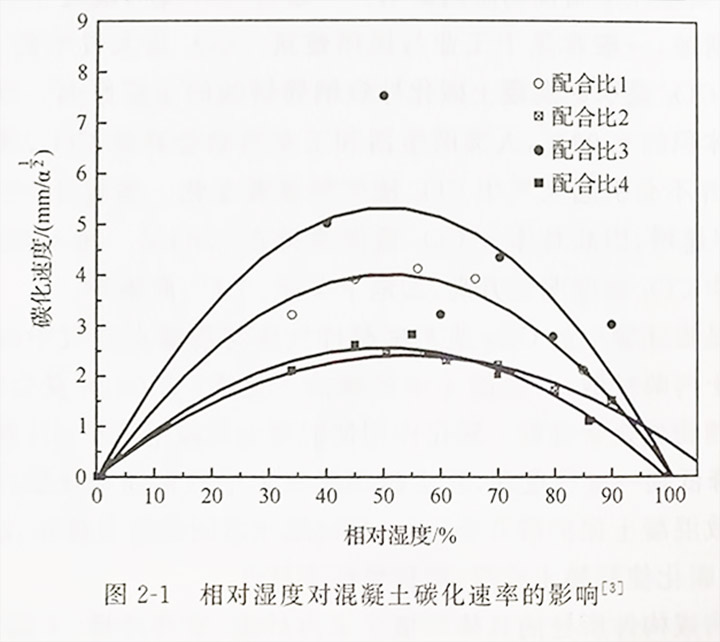
3. Relative humidity of the environment
The environmental humidity has a significant impact on the carbonation rate of concrete. The change in relative humidity determines the degree of saturation of pore water in concrete: if the relative humidity in the environment is too high, the concrete will always be underwater or the humidity will be close to saturation, making it difficult for CO and 0 in the air to diffuse into the interior of the concrete, and carbonization cannot or can only proceed slowly; If the relative humidity of the environment is too low and the concrete is in a relatively dry or low moisture content state, although the diffusion rate of CO is fast, the carbonation rate is also slow due to insufficient water supply required for carbonation reaction. Domestic and foreign carbonation data indicate that the relationship between carbonation rate and relative humidity follows a parabolic curve, as shown in Figure 2-1. When the relative humidity is between 40% and 60%, the carbonation rate is the fastest, but the steel bars in the concrete are hardly corroded at this time. When the relative humidity is between 50% and 80%, there is often a larger carbonation rate. Therefore, the most dangerous condition in the general environment is dry wet alternation. Cahyadi and Uomoto's research shows that when the relative humidity of the environment changes from 50% to 30%, even if the exposure time is quite long, the carbonation rate of concrete does not slow down. There is a certain correlation between relative humidity and the intensity and frequency of precipitation, usually in areas with abundant precipitation
The service environment in Chapter 2 is also relatively large. Therefore, in terms of zoning, it is also feasible to consider the impact of precipitation and relative humidity together.
4. Wind
Wind pressure and direction both have an impact on carbonization [5-7], and wind pressure accelerates carbonization. The influence of wind pressure in the atmospheric environment on the durability of concrete is not only manifested in accelerating the diffusion of acidic gases inside the concrete, but also in accelerating the penetration of moisture, oxygen, and other harmful gas impurities (such as chloride ions) into the concrete. The impact of wind on carbonization is quite complex. It not only accelerates carbonization on the windward surface, but also hinders the carbonization process due to the splashing of precipitation. Therefore, it is currently difficult to fully reflect the influence of wind on carbonization in the carbonization model.
5. Influence of chloride ion concentration
In the practical use of reinforced concrete structures, the carbonation of concrete and the erosion of chloride ions are often intertwined. Research has shown that the carbonation depth of concrete decreases with the increase of chloride ion content, and the presence of chloride ions will maintain a high humidity inside the concrete, hindering the progress of concrete carbonation; However, research also shows that although chloride ions have a hindering effect on concrete carbonation, if the carbonation of concrete and the erosion of chloride ions work together, it will lead to more severe corrosion of steel bars inside the concrete.
|
|